MEET NINA
Your noninvasive
neuromodulation assistant
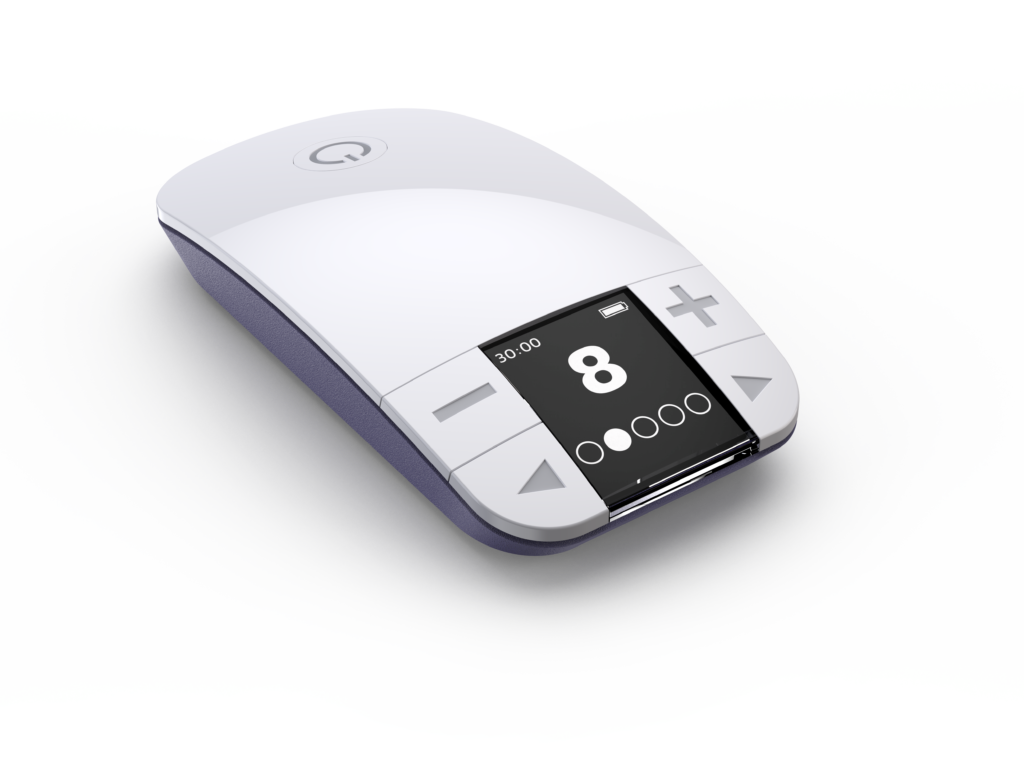
Drug-Free, Noninvasive, At-Home Therapy For OAB
For patients who don’t want to take another pill and aren’t ready for surgery, NiNA* may be an ideal alternative to medication. NiNA uses proprietary technologies to optimally target and activate the saphenous while maintaining user comfort. This simple, discreet system for the treatment of OAB requires just 30 minutes of stimulation and is designed to deliver significant, durable symptom reduction all done in the convenience of your own home.
85%
Physicians would prescribe NiNA to 85% of their OAB patients 1
3 to 1
patients prefer NiNA to medications 3 to 12
85%
Physicians would prescribe NiNA to 85% of their OAB patients 1
3 to 1
patients prefer NiNA to medications 3 to 12
Initial nSAFN clinical experience demonstrates efficacy on-par with published results for medications and other competing therapies
Noninvasive Saphenous Neuromodulation (nSAFN) has been studied in 3 feasibility studies to date at 7 centers in the US and Canada.3

78%
OAB responders3,4

65%
UUI responders3

85%
UUI reduction in UUI
responders3

28%
of patients were Dry3
NiNA vs Competitive Therapies

Medications
Significant side-effects
NO serious adverse effects

Percutaneous Tibial Neuromodulation (PTNS)
Weekly office visits
Done at home

Sacral Neuromodulation (SNS)
Surgery required
Completely noninvasive
Botulinum Toxin
Bladder wall injections
Completely noninvasive, done at home
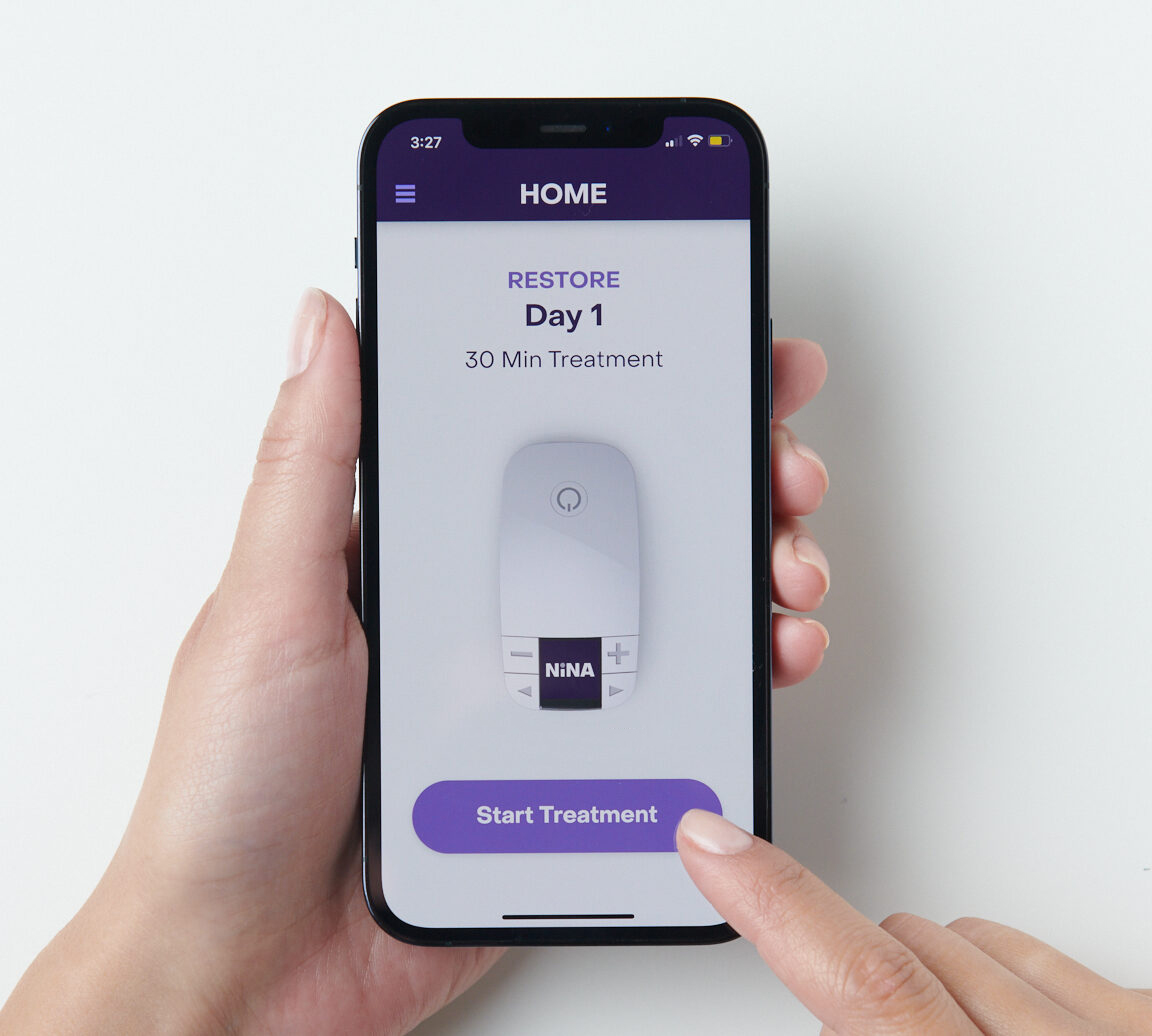
the Future of NiNA
An Intelligent Companion App and Ecosystem
1Primary market research study, conducted by third party Q2-2021, data on file.
2Primary market research study, conducted by third party Q1-2021, data on file.
3Feasibility clinical outcomes, data on file.
4Defined as >=50% reduction or return to normal voiding. UUI = urinary urge incontinence.
5Brazzelli, M., et al. (2006). Efficacy and safety of sacral nerve stimulation for urinary urge incontinence: a systematic review. The Journal of Urology, 175(3 Pt 1), 835–841.
6NiNA Care is currently in development and has not received market authorization for sale in the US, Canada, or any other geography.

